
Building for Success on a Strong Pharmacokinetic Foundation
Pharmacokinetics (PK) tracks a medicine’s fate over time as it goes through absorption and elimination from the body. It’s a concept that is often overlooked during drug development amidst the understandable focus on efficacy and safety.
But this view is misguided. As any drug hunter will confirm, the pharmacokinetic properties of a drug form an integral part of its efficacy and safety. Think of pharmacokinetics not as a dreary cellar in the architecture of a drug’s success, but the foundation upon which the whole thing is built.
This importance is most evident in a drug-target combination that requires exquisite specificity, such as our investigational antibody SNS-101 and the VISTA receptor. Ubiquitously expressed on myeloid cells outside the tumor microenvironment (TME), VISTA has historically been a challenging target for immuno-oncology. Initial attempts to drug VISTA broadly resulted in high off-tumor/on-target activity, leading to dose-limiting cytokine release syndrome (CRS) and depleting the amount of drug reaching the TME. The issue was a poor pharmacokinetic and off-tumor/on-target binding profile presenting a functional hurdle on the path to good efficacy and safety. As we learned quickly from these early efforts, good pharmacokinetics is a prerequisite to any efficacious and safe antibody with good specificity.
Sensei’s fierce drive to transform VISTA from an undruggable target into a successful immuno-oncology target has paid off since those early days. We responded to the PK challenge with a conditionally active approach for SNS-101. By leveraging the low pH of the TME, SNS-101 is designed to actively bind only where needed, potentially reducing off-tumor effects and the consequent CRS and rapid depletion of the antibody.
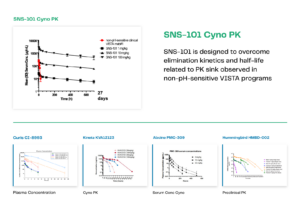
The SNS-101 program received IND clearance from the FDA in April 2023 and we will eventually see if its pharmacokinetic properties translate into efficacy for patients. But we believe the proof of principle has already been achieved in terms of PK, given both GLP and non-GLP data in non-human primates (Figure). Measured over 28 days, three doses of SNS-101 between 1 and 100 mg/kg showed linear elimination kinetics, a hallmark of a favorable PK profile, indicating substantially longer half-life than the non-pH-sensitive control compound in the study. The results contrasted starkly from other VISTA programs, with the downward trending lines in those PK curves demonstrating a ‘PK sink’ in which the drug is eliminated quickly, just hours in some cases, from circulation through absorption by the many off-tumor VISTA-positive cells. This positive result has been the foundation of, and the leading indicator of, the favorable efficacy and safety profile that we observed in subsequent preclinical studies of SNS-101.
More information on SNS-101 can be found in the 2023 Sensei Company Presentation.
The strength of these preclinical data have brought Sensei into the clinic this year. We expect that SNS-101’s superior PK profile will allow us to test higher doses and less frequent dosing in patients than competing VISTA programs. Because SNS-101’s distribution is specific to the TME, we believe the likelihood of adverse effects due to VISTA binding in the periphery may be significantly reduced. Add it all up, and the combination of our preclinical PK, efficacy and toxicity data support the design of a potentially more efficient Phase 1/2 clinical trial for SNS-101, in which combination therapies using other checkpoint inhibitors could be introduced earlier in the trial than otherwise. To wit: the starting dose of 0.3 mg/kg that the FDA approved for SNS-101 is 60-fold higher than the first attempt to drug VISTA by Janssen in 2016. We’re beginning our journey in the clinic on as clear a path as we could have hoped, thanks to SNS-101’s stellar preclinical data and, more importantly, the teams at Sensei that made those data happen.
We’re nothing if not committed to innovative approaches for solving problems at Sensei, and that applies to our use of metaphors too. Much like the solid foundation of a building, we believe SNS-101’s pharmacokinetic data will enable us to build a strong, enduring and overarching edifice from which we can observe the vista. It’s time to see what the view looks like.
Cautionary Note Regarding Forward-Looking Statements
Any statements contained in this article that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words and phrases such as “believe,” “designed to,” “expect,” “may,” “plan,” “potential,” “will” and similar expressions, and are based on Sensei’s current beliefs and expectations. These forward-looking statements include expectations regarding the development of SNS-101; the potential safety profile of SNS-101; and the potential benefits and efficacy of SNS-101. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the development of therapeutic product candidates, such as the risk that any one or more of Sensei’s product candidates, including SNS-101, will not be successfully developed or commercialized; the risk of delay or cessation of any planned clinical trials of Sensei’s product candidates, including SNS-101; the risk that prior results, such as signals of safety, activity or durability of effect, observed from preclinical studies, including the preclinical studies of SNS-101, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Sensei’s product candidates; the risk that Sensei’s product candidates, including SNS-101, or procedures in connection with the administration thereof will not have the safety or efficacy profile that Sensei anticipates; risks associated with Sensei’s dependence on third-party suppliers and manufacturers, including sole source suppliers, over which Sensei may not always have full control; risks regarding the accuracy of Sensei’s estimates of expenses, capital requirements and needs for additional financing; and other risks and uncertainties that are described in Sensei’s Quarterly Report on Form 10-Q filed with the U.S. Securities and Exchange Commission (SEC) on May 9, 2023 and Sensei’s other Periodic Reports filed with the SEC. Any forward-looking statements speak only as of the date of this article and are based on information available to Sensei as of the date of this article, and Sensei assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise.